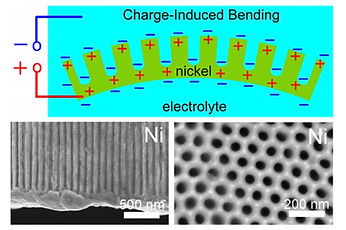
Top shows the schematic of charge-induced bending mechanism in nanohoneycomb Ni actuator. Lower left shows a sectioned view of nanohoneycomb structure, while lower right shows the top view of nanohoneycomb, similar to that of bee hive.
Cyclic voltammatry at different scan rates during actuation. (Indent shows different anodic and cathodic current peaks corresponding to the square of scan rates)
Free-end displacement plotted against scanned potential at various scan rates. Grey strip indicates the linear displacement extrapolation due to electric-double-layer mechanism.
Nanohoneycomb Ni actuator
Synthesis:
The nickel nanohoneycomb actuator in the present study is synthesized using "dual-template method" developed at our laboratory. Contrary to de-alloying method, where only limited type of metals can be used to synthesize nanoporous actuators due the difficulties to synthesize precursor materials (difficult to find two metals forming precursor alloy that are able to form solid solution without segregation), the dual-template method can be applied to any metals as long as it can be electrodeposited onto an electrode. This method is capable of fabricating centimeter-scale samples, and is highly repeatable and of lower costs.
The actuator is a bi-layer nanohoneycomb Ni/bulk Ni. The synthesis of nanohoneycomb Ni happens in several steps. Firstly, a 3D nanohoneycomb template is synthesized by anodizing Al foil to obtain nanohoneycomb anodized aluminum oxide (AAO) with pore size ~ 50nm. Then, an inverse polymer mould is synthesized by thermal mounting onto nanohoneycomb AAO. After etching away the original AAO template, nickel is electrodeposited onto the polymer inverse mould, producing a nanohoneycomb nickel layer which highly resembles the original nanohoneycomb AAO template.



Benefits of nanohoneycomb Ni actuator:
1. Highly ordered nanostrucutre improves macroscopic strain and actuation rates: Drawbacks of nanoporous Au/Pt synthesized with de-alloying method include: (1) randomly oriented nanoligaments generates greater hindrance for ions to diffuse in and out of the structure, hampering the rate of ion diffusion speed and thus the actuation rates. (2) Since the nanoligaments are not aligned along a specific direction, the strain can occur in all different orientations. The strain in neighboring nanoligaments may cancel out one another, resulting in less significant macroscopic strain. In nanohoneycomb actuator, the strain along the parallel nanoporous walls are effectively unified and aggregated, and the highly ordered nanoporous channels generates less resistance for ion diffusion and increases the actuation rate.
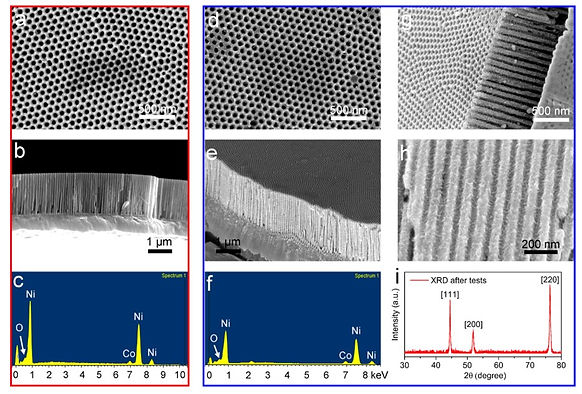
2. Formation of nickel hydroxide layer prevents ligament coarsening: The nanohoneycomb Ni actuator was characterized before and after 500 cyclic actuation loops at various frequencies. The nanohoneycomb architecture was well preserved after the test, without any signs of blocking or collapse of the pore channels. This is because the Ni(OH)2 thin film formed at the surface of the nanohoneycomb is highly stable, preventing the actuator from suffering from nanoligament coarsening, a major concern for nanoporous metal actuators.

3. Lower porosity results in higher Young's modulus and actuation stress: The maximum strain obtained at the lowest scan rate (25mV/s) is 2.5 x 10^(-4) %, whereas the strain at highest scan rate (500mV/s) reduced by half to 1.3 x 10^(-4) %. The relatively low strain compared to other nanoporous metals is due to the low porosity (~23%). The porosity in state-of-the-art nanoporous Pt is as high as 90%. [2] However, as a result of have less pores, we attained a Young's Modulus (E ~ 108 GPa) in the material that is much higher than most nanoporous Pt (~9 GPa) [2] or nanoporous Au (~11 GPa) [3]. Moreover, the current strain rate (~10^(-4)%/s) is an order of magnitude higher than nanoporous Pt operating in the capacitive-double-layer region (~10^(-5)%/s) owing to the faster ion diffusion rate and parallel signaling feature facilitated by the nanohoneycomb structure.
Reference:
[1] Cheng C, Ngan HW. 2015. Reversible Electrochemical Actuation of Metallic Nanohoneycombs Induced by Pseudocapacitive Redox Processes. ACS nano, 9(4):3984-3995
[2] Weissmüller, J; Viswanath, R. N, Kramer, D, Zimmer, P, Würschum, R, Gleiter, H. 2003. Charge-Induced Reversible Strain in a Metal, Science, 300:312–315
[3] Biener, J, Hodge, AM, Hamza, AV, Hsiung, LM, Satcher, JH. 2005. Nanoporous Au: A High Yield Strength Material. J. Appl. Phys., 97:024301-1–024301-4
Before actuation
After actuation

Dual-actuation mechanisms:
Two actuation mechanisms were identified in nanohoneycomb nickel through the analysis of free end displacement versus applied voltage. While the actuator was immersed in 1 M NaOH actuated by a positive potential scan between -0.1V and 0.5V, the free-end displacement first increased linearly with the potential but suddenly increased much faster between 0.15V and 0.5V. The stroke at lower potential (-0.1V ~0.15V) only contributed to 33% of the total strain, while the displacement at higher potential (0.15V-0.5V) dominated most of the strain (67%).
1. Charging/discharging of capacitive electrochemical double layer:
The strain occurring between -0.1V~0.15V (displayed in the grey region in the figure below) is due to capacitive charging of electrochemical double layer, and the actuation strain is linearly proportional to the applied voltage. This is the same actuation mechanism as in nanoporous Au/Pt actuator with a "clean" metal surface.
2. Redox reaction between nickel hydroxide/nickel oxyhydroxide: The second actuation mechanism which contributes to a greater portion of the strain (67%) while the applied voltage is in te range of 0.15V - 0.5V was hypothesized as the redox reaction of Ni(OH)2 <-> NiOOH, or Ni(II)<->Ni(III). When Ni(OH)2 oxidizes into NiOOH, the unit cell volume shrinks by more than 15% and this results in contraction of the actuator in the direction towards the nanohoneycomb Ni. This hypothesis is confirmed in several experimental aspects.
Firstly, the nanoporous walls roughened after series of actuation tests as revealed by SEM images. The roughening is believed to be caused by a thin film of nickel hydroxide layer formed at surface. The oxygen content of in the nanohoneycomb also increased from 2.6 %wt to 4.1% wt after actuation tests, further confirming the existence of Ni(OH)2 thin film. Secondly, through the lower right figure (cyclic voltammatry during actuation), anodic and cathodic current peaks are identified at 0.4V and 0.1V respectively during positive and negative voltage scanning. This voltage level coincides with the oxidation and reduction potential of the redox pair, Ni(OH)2 and NiOOH.
This is the first time redox reaction is employed for electrochemical actuation, possibly due to the lack of metal species that exhibit obvious redox reaction during actuation.
Nanoporous nickel appears in bright gold due to its nanostructure. Normal Ni is silver in color and is shown on the right for comparison.
Microstruture:
The straight nanopore channels in the nanohoneycomb highly resemble skeletal muscles fibres. Human muscles consist of bundles of muscle fibres arranged in parallel directions, similar to the nanoporous walls in the nanohoneycomb Ni. The actuation in muscles are quick and significant because parallel fibres are signaled and actuated simultaneously, where the individual strain in each fibres adds up to induce large strain -- also known as the "parallel signaling" feature. Likewise in the present work, the contraction in each pore unit of the nanohoneycomb effectively adds up to generate a large macroscopic strain (see below).
